Raffaella Mastrocola
University of Torino
Italy
Title: The Advanced Glycation End-Products/SREBP1c axis as a new pathogenic mechanism in diet-induced metabolic disorders
Biography
Biography: Raffaella Mastrocola
Abstract
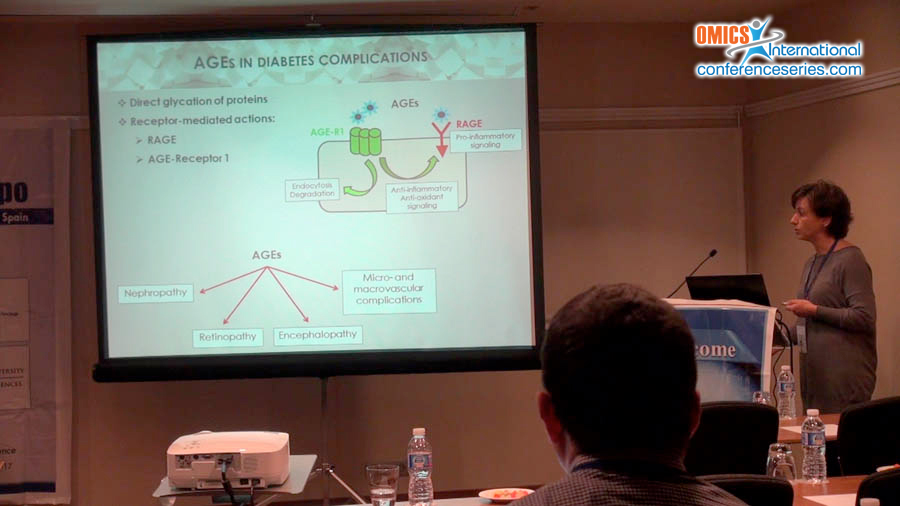
The worldwide epidemic rise in metabolic disorders is tightly linked to excessive dietary intake of saturated fat and sugars. Although Advanced Glycation End-Products (AGEs) are toxic compounds well-known for their roles in type 1 diabetes complications, recent studies indicate their diet-induced accumulation in several dysmetabolic conditions, as obesity, insulin resistance, and type 2 diabetes. Consistently, we have recently reported in different animal models of diet-induced metabolic disorders the associative link between AGEs and the dysregulated activation of the lipogenic transcription factor SREBP1c. In particular, we have evidenced a double mechanism by which carboxy methyllysine (CML), the most studied AGE, interferes on SREBP1c activation by the direct glycation of its activating protein SCAP and by the reduction of the SREBP1c inhibitor SIRT-1. As consequence of SREBP1c overactivation, a marked lipid deposition was found in liver and skeletal muscle of mice fed high-fat and high-sugar diets. In addition, recent studies highlighted new transcriptional roles for SREBP1c in the development of cell damage and metabolic impairment. Specifically, we analyzed in different target tissues of dysmetabolism, as liver, skeletal muscle and brain, the effect of SREBP1c overactivation on selective pathways involved in the regulation of antioxidant and inflammatory response and structural and metabolic adaptation. Finally, our most recent findings evidenced that the inhibition of CML production by the administration of the anti-glycative compound pyridoxamine to mice fed a high-fructose diet was able to prevent both SREBP1c activation and SREBP1c-depending signaling impairment.