Day 2 :
Keynote Forum
Gerald J Prudhomme
University of Toronto, Canada
Keynote: Treatment Of Human Pancreatic Beta Cells With A Combination Of Gamma-Aminobutyric Acid (Gaba) And Glp-1 Ameliorates Cell Survival And Proliferation
Time : 10:-00-10:45

Biography:
Gerald J Prud'homme is a Professor in the Department of Laboratory Medicine and Pathobiology at the University of Toronto, and Clinician-Scientist at St. Michael’s Hospital, Toronto. He received his MD degree from the University of Ottawa, Canada (1977) and subsequently specialized in Pathology. He is a Research Fellow at the Institute of Immunology, University of Toronto, the Scripps Research Institute and the McGill Cancer Centre (McGill University, Montreal). Subsequently (1985-2002), he worked as a Scientist and Pathologist in the Department of Pathology, McGill University. His main research interests are in the areas of immunotherapy of autoimmune diseases and cancer therapy.
Abstract:
An effective therapy for type 1 diabetes (T1D) requires the protection of pancreatic beta cells against autoimmunity (immunosuppression and/or anti-inflammatory activity) and beta-cell proliferation or regeneration. No current treatment achieves both goals in a clinical setting. The incretin hormone GLP-1 is effective in the treatment of type 2 diabetes (T2D), but not T1D. Recent studies by us and others have shown that GABA protects beta cells against autoimmune injury and induces their regeneration in mice. In this study, we investigated the effects of these drugs on human islets cells, and compared their response to rodent insulinoma cell lines. We found that GABA increases SIRT1 and Klotho (mRNA and protein), and prevents apoptosis induced by high glucose levels or inflammatory cytokines. Importantly, both Klotho and SIRT1 inhibit the activation of NF-kB. The NF-kB inflammatory pathway provokes beta-cell apoptosis, such that its blockade is protective. We show that a GLP-1 receptor (GLP-1R) agonistic drug ameliorates the effects of GABA in some assays. However, we observed that a GLP-1R agonist does not stimulate human beta-cell proliferation, whereas GABA does promote proliferation. We conclude that GABA, especially when combined with GLP-1, effectively protects human beta islet cells against glucotoxicity and other injuries, and that GABA (but not GLP-1) stimulates their proliferation. These observations suggest that GABA+GLP-1 therapy will be effective in human T1D, due to a combination of anti-apoptotic, anti-inflammatory and proliferative/regenerative effects.
Keynote Forum
Moorkath Nandakumaran
University of Kuwait, Kuwait
Keynote: Maternal-Fetal Transport Kinetics Of 3-O-Methyl Glucose In Diabetic Model Perfused Human Placenta: In Vitro Study
Time : 10:45-11:30

Biography:
Moorkath Nandakumaran obtained his Doctorate degree in Reproductive Physiology from University of Paris VI in 1979 and has done his specialization in maternal-fetal transport of nutrients and drugs across the human placenta as well as experimentally induced diabetic rats for the past many years. He has been an Invited Speaker for more than six international conferences and has developed human placental perfusion models for study of pregnancy related conditions such as pre-eclampsia and diabetes. He has published nearly 40 papers in international journals related to the area of maternal-fetal transport in health and disease states.
Abstract:
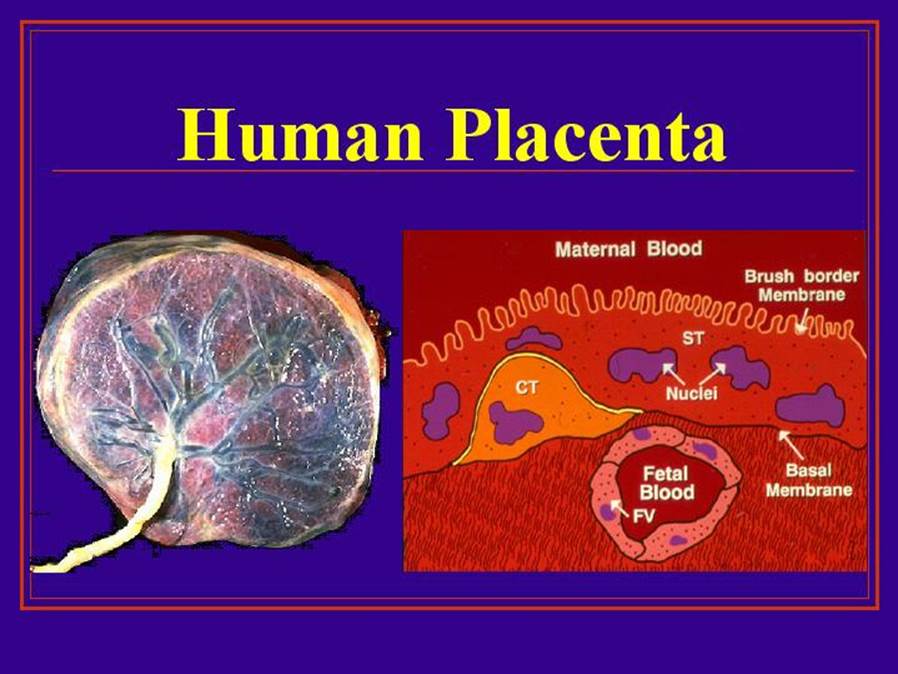
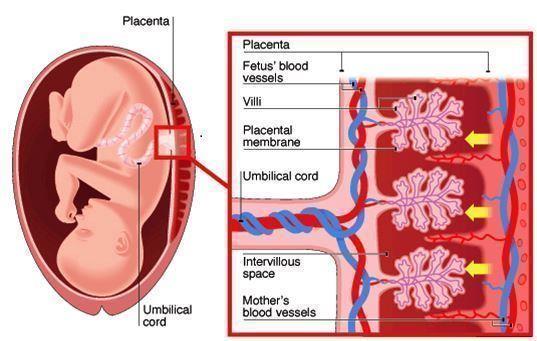
Previous studies from our laboratory had shown that maternal-fetal transport kinetics of model amino acids such as alpha-aminoisobutyric acid and L-Leucine and model fatty acid, palmitic acid were compromised in diabetic model placental lobules perfused in vitro. This study was meant to explore whether transport kinetics of a model hexose, 30-methyl glucose were altered in diabetic model human placental perfusions in in vitro conditions. Human placentae were collected immediately after delivery and perfused with NCTC culture medium buffered with Earles salt solution perfusate. After wash-out period of about ten minutes, 14-C labeled 3-0-methyl glucose (specific activity: 59 mCi/mmol, Amersham, UK) along with tritiated water (specific activity 5 mCi/mmol, Amersham, UK) as reference marker were then injected as a single bolus (100 ml) into the maternal arterial circulation of perfused placental lobules and perfusate samples collected from maternal and fetal circulations over a period of 5 minutes. In another series of experiments, glucose concentration in maternal arterial perfusate was doubled to about 11 mmol/L to mimic a moderate hyperglycemic diabetic state and prefusions were done for five minutes in the control euglycemic series. Concentration of 14C and 3H labeled study and control substances in perfusate samples in control and diabetes model perfusions was assessed by scintillation spectrometry. Transport kinetics of 3-O-methyl glucose and tritiated water were computed using established permeation parameters. Differential transport rates of 3-O-methyl glucose and tritiated water in 8 perfusions differed significantly (Student's t-test; p<0.05) for all transport fractions studied in control perfusions and in perfusions from five diabetic model perfusions. Transport fraction index of 3-O-methyl glucose compared to reference marker averaged 29.9% in control perfusions (n=8) and 33.90% in diabetic model perfusions pregnancies (n=5), respectively. The difference observed in TF index of 3-O-methyl glucose compared to tritiated water in control and diabetic groups were not statistically significant (Student's t-test, p>0.05). Our studies show for the first time that transport behavior of a model hexose, 3-O-methyl glucose is not significantly different or compromised in diabetic model perfusions. We are unable to speculate whether transport kinetics of hexoses could be altered in diabetic pregnant women in vivo state with moderate hyperglycemia as in this study or in diabetic state with greater glucose load than 11 mmol/L as in severe diabetic state.
Keynote Forum
H Henry Dong
University of Pittsburgh, USA
Keynote: Foxo Transcription Factors In Type 2 Diabetes
Time : 11:45-12:30

Biography:
H Henry Dong is the Professor of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh, USA. He is a member of American Diabetes Association. He is serving as an Editorial Board Member of Journal of Biological Chemistry, Molecular Metabolism, and Journal of Diabetes and its Complications.
Abstract:
The forkhead box O family consists of FoxO1, FoxO3, FoxO4 and FoxO6 proteins in mammals. They share a common structural motif, namely the “forkhead box” or “winged helix” domain that is responsible for binding to chromatin DNA in the nucleus of cells. FoxO proteins act as nuclear transcription factors that mediate the inhibitory action of insulin or insulin-like growth factor on key functions in diverse pathways including cell metabolism, proliferation, differentiation, oxidative stress, cell survival and senescence, autophagy and aging in mammals. Genetic mutations in FoxO genes or abnormal expression of FoxO proteins are associated with metabolic disease, cancer or altered lifespan in humans and animals. My laboratory focuses on the studies of FoxO1 in glucose and lipid metabolism. FoxO1 is abundantly expressed in the liver and its transcriptional activity is tightly regulated by insulin. Insulin inhibits FoxO1 activity via a distinct mechanism by altering its subcellular redistribution. We showed that insulin signaling bifurcates at FoxO1 in the liver to govern two metabolic pathways, namely gluconeogenesis and very low-density lipoprotein (VLDL) assembly. This effect helps synchronize hepatic insulin signaling to simultaneously adjust the rates of hepatic glucose production and VLDL secretion in response to nutrient availability. Such FoxO1-dependent mechanism seems pivotal for the liver to rapidly adapt to metabolic shift between fasting to feeding states for maintaining normal glucose and lipid homeostasis. Our studies provided mechanistic insights into how FoxO1 orchestrates insulin action on hepatic glucose and lipid metabolism in healthy individuals, and how FoxO1 dysregulation, resulting from insulin resistance, contributes to the dual pathogenesis of hyperglycemia and hyperlipidemia in obesity and type 2 diabetes. We screened for small molecule drugs against FoxO1, demonstrating that pharmacological FoxO1 inhibition translated into a significant beneficial effect on glucose and lipid metabolism in mice with diabetic dyslipidemia. Our studies characterize FoxO1 as a potential therapeutic target for improving blood glucose and lipid profiles in type 2 diabetes.